For more on KEI’s work on COVID-19, see keonline.org/coronavirus.
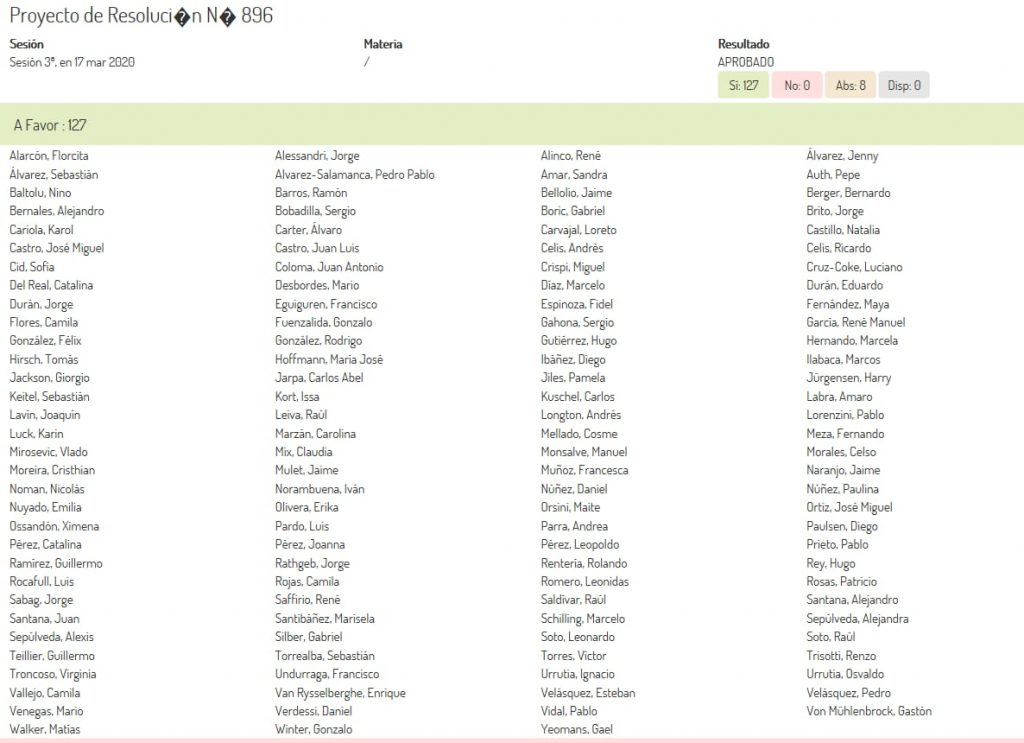
Today, with an overwhelming 127 votes in favor and 0 votes against, the Chilean Chamber of Deputies approved a strong resolution asking the Chilean Government to declare that there is justification for compulsory licenses for vaccines, drugs, diagnostics, devices, supplies, and other technologies useful for the surveillance, prevention, detection, diagnosis and treatment of people infected by the coronavirus in Chile.
The resolution also has a strong transparency mandate, asking the Minister of Health to request the World Health Organization (WHO) Global Observatory on Health R&D to collect information on the costs associated with health technologies related to COVID-19.
This is a PDF of the resolution:
A KEI translation of the full resolution is available here. The following are the operative elements of Resolution 896, translated into English by KEI.
-
-
- RESOLUTION FOR THE GRANTING OF NON-VOLUNTARY LICENSES REFERRED TO IN ARTICLE 51º Nº 2 OF INDUSTRIAL PROPERTY LAW Nº 19.030 TO FACILITATE ACCESS AND AVAILABILITY OF MEDICINES AND TECHNOLOGIES FOR THE PREVENTION, TREATMENT AND CURE OF CORONAVIRUS COVID-19
-
III. THAT, THEREFORE, THE H. CHAMBER OF DEPUTIES, AGREES THE FOLLOWING DRAFT RESOLUTION:
1.- DECLARE: That pursuant to this background, the coronavirus epidemic worldwide and in our country, and its risks to the health of the Chilean population, in accordance with the aforementioned, constitute sufficient justification for the granting of the non-voluntary licenses contemplated in article 51º No. 2 of Industrial Property Law No. 19.039 to facilitate access to vaccines, drugs, diagnostics, devices, supplies, and other technologies useful for the surveillance, prevention, detection, diagnosis and treatment of people infected by the coronavirus virus in Chile, for public health reasons and/or national emergency, as provided in international laws, particularly the Doha Declaration on the TRIPS Agreement and Public Health.
2.- REQUIRE: The Minister of Health, Mr. Jaime Mañalich Muxi, to declare the existence of Public Health reasons for the granting of non-voluntary licenses provided in Article 51º No. 2 of Industrial Property Law No. 19.039, regarding all patent applications and issued patents related to vaccines, drugs, diagnostics, devices, supplies, and other technologies useful for the surveillance, prevention, detection, diagnosis and treatment of persons infected with the coronavirus SARS-CoV-2, por public health grounds.
3.- TO REQUIRE: The Minister of Health, Mr. Jaime Mañalich Muxi, to instruct the corresponding ministerial departments, as well as the health services and the Supply Center, to report the vaccines, drugs, diagnostic tests, supplies, equipment and others technologies that are projected will be required as an essential, for the purposes of the determination by the National Institute of Industrial Property of the the existence of patents or other industrial rights that restrict their importation or national production.
4. TO REQUIRE: the Minister of Health to request the World Health Organization (WHO) Global Observatory on Health R&D to collect information on the research and development costs directly associated with vaccines, drugs, diagnostics, devices, supplies, and other technologies useful for the surveillance, prevention, detection, diagnosis and treatment of COVID-19, including the investments made by public sector institutions, private sector institutions, and charities.
Commentary from Chile on the resolution:
Deputy Giorgio Jackson.
-
- “Today we approved a draft resolution for the Government to declare COVID-19 as a public health problem for the purposes of eventual non-voluntary licenses over vaccines or treatments that may be needed to fight the pandemic, Hopefully all governments do it!”
Luis Villarroel, Director of Corporación Innovarte (advocacy group that played a key advocacy role in support of Resolution 896).
-
- “This is an important step towards strengthening available legal tools for access to technologies that are useful for fighting this pandemic in Chile. And, improving transparency through the Global Observatory on Health R&D, as mandated in the resolution, would allow us to better understand the costs of developing these technologies and what roles are being played by each actor.”
This was the vote: